edcoble
Well-known member
- Joined
- Jul 11, 2023
- Threads
- 4
- Messages
- 47
- Reaction score
- 26
- Location
- Fort Worth, Texas Ed
- Vehicles
- 2023 Ford Lightning Lariat (ER)
- Occupation
- Retired/former lawyer/former pastor
Thanks, Dan.
Sponsored
I would disagree on that one 1000%. Just because so 🫏The safest speed to drive is that of surrounding traffic regardless of the posted speed limit. Differential speed (higher or lower) is less safe.
No, there is a 3K foot elevation change going the other way and there is usually a strong headwind. I usually charge in Wagon Mound but of course that adds 60 miles. When the Superchargers open up I will be able to charge in Clayton and drive straight through.Dan, another question: did you drive the other direction, from Amarillo to Trinidad? If so, were you able to drive it without charging?
There are two new Tritium chargers at the Phillips 66 station, these are not the Sierra Grande chargers that have been promised for so long. The chargers look ready go but I don’t know what network they will be on and I don’t know what the power rate will be. I will be going through Des Moines this weekend and I will take a closer look at the chargers.Thanks! I was afraid of that. I've been watching for that promised Des Moines installation but haven't seen any actual work occurring yet.
My limited understanding (I did not stay at a Holiday Inn Express last night) is that the available power output might be less when super cold, but the stored energy is not affected.
The kWh’s are all there, they’re just less effective/effecient.
But when the battery warms up it somehow gains that energy back? The physics of this is a little out of my wheel house but something that does interest me. While I was on vacation over the holidays, I was plugged into a 120V outdoor outlet to top up. I was at 100% indicated SOC one morning when we left to go to lunch, about 10 miles away. It was in the 30's overnight and almost 60 after lunch. Despite driving 10 miles (50-60mph, slight elevation gain), I had 100% SOC upon turning the truck on to go home. Where did that extra energy come from? Does the battery absorb electrons from the radiant heat of the parking lot? Or is it from the energy that was already stored in the battery but didn't become available until the battery warmed up? The latter makes more sense but seems to contradict the claim that batteries don't store as much energy when cold.Bit of a bump here but I wanted to expand on this point here with some actual data and the temps have been too warm... the "stored energy"/kWh is not the same when the temperature of the pack is lowered... temperature lowers the voltage of a battery, which directly reduces the amount of energy available. Heat is energy, so if you reduce the temperature of the battery you are removing energy from the electrons (demonstrated via voltage drop, since voltage is basically the 'potential energy' of the electrons).
The voltage of the pack will increase, so the capacity will increase. You have the same number of electrons, but they have more energy. This is only a net positive for you if you have an external source of energy for the heat, though.But when the battery warms up it somehow gains that energy back?
Not quite the right wording. The energy isn't removed directly from the electrons but from the effect of temperature on the chemical reaction(s) that produces the electrons.Heat is energy, so if you reduce the temperature of the battery you are removing energy from the electrons (demonstrated via voltage drop, since voltage is basically the 'potential energy' of the electrons).
Introduction
Source: Temperature-dependent interphase formation and Li+ transport in lithium metal batteries | Nature CommunicationsLithium-ion batteries (LIBs) operating at a low temperature are highly wanted in the cold seasons or locations for different applications such as electric vehicles, submarines, and airplanes. Anxiety rises for the reduced battery capacity or drive range but the increased safety issues at the low temperature, which dampens the enthusiasm for widespread usage of LIBs. This is largely due to the slowed-down kinetics of Li ions associated with movement and reactions, resulting in increased resistance, reduced power capability, and even dendritic Li plating1,2,3,4. Thoroughly understanding the influence of temperature on the underlying microstructure and performance of the battery is essential to solving the kinetic bottlenecks and achieving high performance at a low temperature.
Fair point... My post was already longer than I meantNot quite the right wording. The energy isn't removed directly from the electrons but from the effect of temperature on the chemical reaction(s) that produces the electrons.
Here's a good explanation of why, when low temperature causes the internal resistance of a Li-ion battery to increase, the voltage across the load decreases. Basically, because the voltage the chemical reaction can deliver will be distributed as the voltage internal to the battery plus the voltage across the load. When the voltage drop internal to the battery rises because of increased resistance, the voltage available to the load has to drop.Not quite the right wording. The energy isn't removed directly from the electrons but from the effect of temperature on the chemical reaction(s) that produces the electrons.
Source: https://qr.ae/pKx0I5Consider the actual circuit of the above battery feeding a load resistance RL.
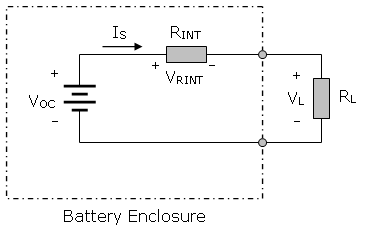
By Ohm’s Law, Is = Voc/Rt = Voc/(Rint + RL)
The voltage across the load, VL = Is ٠ RL = Voc ٠ RL / (Rint + RL)
The voltage “drop”, is the voltage lost across the internal resistance of the battery.:
Vdrop = Voc - VL = Is ٠ Rint = Voc - [Voc ٠ RL/(Rint + RL)]
or Vdrop = Voc [ 1 - RL/(Rint + RL)]
As you can see, the higher the internal resistance of the battery (Rint), the greater the voltage drop will occur across Rint for a given load current Is, and the less voltage you’ll have available across the load (VL). Similarly, the smaller the load resistance RL, the larger effect the internal voltage drop across Rint will have on the voltage available across the load (VL).
I was tempted to show you how to solve the above relationship for Rint, the internal resistance of a battery by (a) measuring its open circuit voltage Voc, and then (b) its terminal voltage VL when you connect a load resistance RL across the battery, but I suspect that if you want that information, you can readily do the algebra yourself.